Arsenic is widely distributed in nature, air, soil environment, water, animal bodies, plants contain trace amounts of inorganic arsenic, seafood and aquatic products also contain trace amounts of organic arsenic, inorganic arsenic toxic side effects are much higher than organic arsenic. Arsenate is toxic and harmful, and can be used as insecticide. Due to the widespread use of arsenic-containing pesticides, the pollution of arsenic to the environment is becoming more and more serious. Arsenic is mainly harmful to human health in the form of compounds, and its compounds are quite complex. After entering the human body, it causes abnormal liver and kidney function and immune function suppression, so that the balance of oxidation and antioxidant system in the patient's body is disturbed, and then lead to cancer. Arsenate, arsenite and bromate have great toxic side effects, which pose a great threat to human health and natural ecological environment, and gradually get people's attention. The limits of arsenic, chlorate and bromate in drinking water are 0.01, 0.7 and 0.01mg/L, respectively, according to the World Health Organization and the Sanitary Standard for Drinking Water. Ion chromatography can be used for simultaneous detection, which is simple and sensitive.
Under different pH conditions, there are several forms of H2AsO4-, HAsO42- and AsO43-. When conventional carbonate is used as eluent, As(v) will appear three peaks due to protonation, overlapping with other anion peaks, which will bring certain interference to the analysis. If NaOH is added to the eluent, the above situation will be avoided.
It is well known that the detector requires a suppressor to deduct the background conductance of the eluent, for some anions with PKa values >7, the background conductance is too low to be detected, the PKa of As(V) is <7, can be directly conductance detection, and PKa of As(III) is 9.22, should not be used conductance detection. Therefore, arsenate can be directly detected, arsenite can not be directly detected, and the detection of arsenite can be detected by oxidation of hydrogen peroxide to arsenate.
Experiment condition
Chromatographic column: Anion column PRA -16
Eluent: Na2CO3 + NaOH
Flow rate: 1.2mL/min
Column temperature: 36.5℃
Injection volume: 100μL
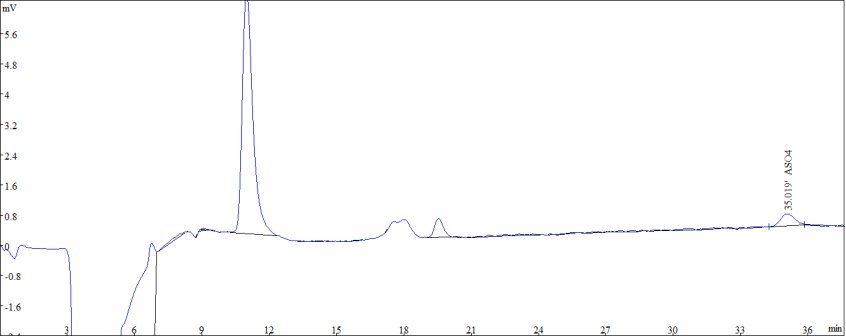
Figure 1: Standard sample diagram of 10 μg/L ASO43-
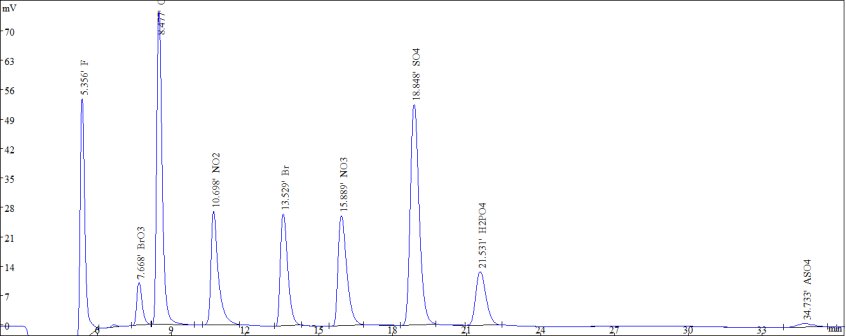
Figure 2. Standard samples of common anions and bromate and arsenate





