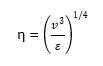
The choice of stirrer type, stirring speed and aeration mode (jet/surface aeration) determines the fluid flow pattern in a conventional stirred tank bioreactor. Typically, the fluid flow is turbulent, with a Reynolds number (Re) ≥ 4000, which can be calculated using the following equation:
Re = (ρNd2)/μ
where ρ is the density of the fluid, N is the stirring speed, d is the diameter of the stirring paddle, and μ is the fluid viscosity.
The formation of turbulent vortices is a unique feature of fluids in agitation. The turbulent kinetic energy contained in the fluid in agitation forms large vortices that break up into smaller vortices and dissipate energy into the fluid in the form of heat. This is a continuous flow of energy from large to small vortices, where the kinetic energy is converted into heat in the form of minimal vortices on the microscopic scale, called Kolmogorov microscopic vortices(η)
The calculation formula is:
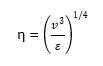
where *Kolmogorov turbulence theory is a set of assumptions that small-scale structures are statistically homogeneous, isotropic, and independent of large-scale structures. The source of energy at large scales is shear or convection. kolmogorov theory describes how energy is transferred from larger to smaller vortices; how much energy is contained in a vortex of a given size.
Shear stress
As previously mentioned, mammalian cells are large and fragile and lack protective cell walls. Therefore, small adverse culture conditions within the bioreactor can be lethal and may force apoptosis or necrosis. It can also show sub-lethal effects such as changes in doubling time, cell activity, oxygen uptake rate (OUR), cell morphology and cell-specific productivity. Preventing premature cell death has therefore become a central issue in bioprocessing.
Various factors, such as changes in the surrounding environment due to lack of essential nutrients, accumulation of by-products, oxygen limitation at high cell densities and changes in pH, temperature or osmotic pressure during the batch run, contribute to overall cell death. In addition, shear effects due to paddle agitation and bubble rupture also contribute significantly to cell death by disrupting cell membranes. These changing conditions trigger signalling pathways that drive apoptosis. There are two main types of cell death pathways, namely necrosis and apoptosis.
Necrosis
It is referred to as passive cell death unrelated to ATP. When exposed to external stress, the cell membrane exhibits certain morphological changes such as swelling, membrane blistering, membrane rupture and cell lysis. This is usually caused by the deterioration of the condition of the medium in the batch cycle. This is due to the accumulation of excess toxic metabolites and depletion of nutrients leading to toxic effects on the cells. In addition to this, bubble rupture and mechanical damage can lead to disruption of cell membrane integrity, which increases the permeability and release of intracellular material.
Apoptosis
Apoptosis is a form of programmed cell death that plays a major role in biological processes, particularly in the cell death of CHO cells (80%). It is an active form of cell death in which ATP is used to carry out the processes that ultimately lead to cell death (signal transduction pathway). It is mainly characterised by chromatin condensation, DNA breakage and cell shrinkage. Apoptosis is triggered by two pathways, primarily the cysteine-containing aspartate-directed protease called cysteinase and the expression of the BCL2 gene, which contains several checkpoints that regulate apoptotic cell death.
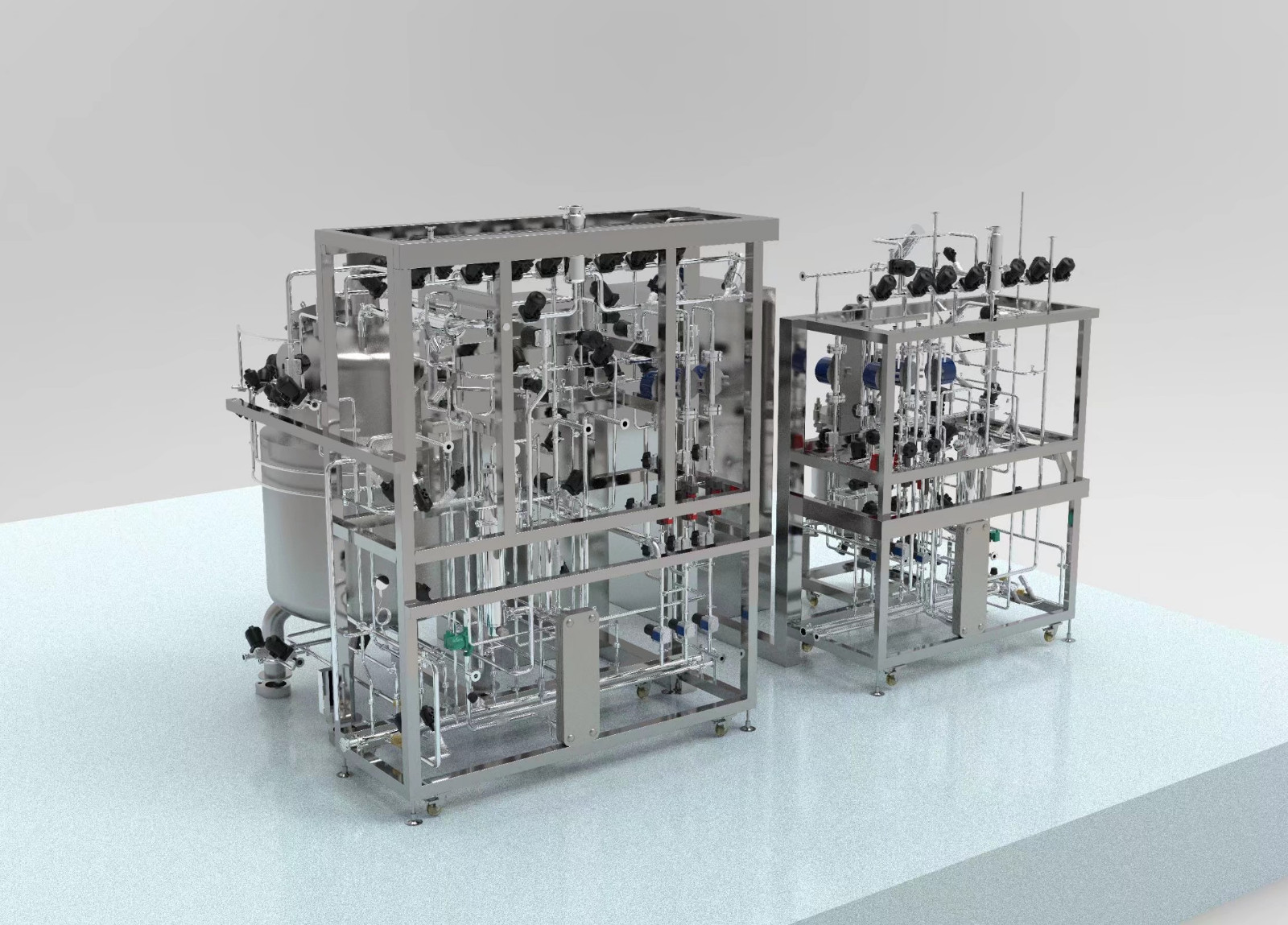
As you can see, cell culture is a complex process that requires a combination of factors to be considered and optimised in order to achieve optimal cell culture performance, including cell density and cell activity, followed by optimal product yield per volume and cell-specific yield. YOCELL has in-depth bioreactor engineering and cell culture process development experience, offering everything from multiplex parallel bioreactors and benchtop scale glass reactors to 2,000 L disposable stirred tank bioreactors for mammalian cell culture processes, as well as complete technical advice and operational support from process development, scale-up and full GMP operation to help you achieve optimal process results and speed up the process.
Reference:
A.W.Nienow, Hydrodynamics of Stirred Bioreactors, Appl. Mech. Rev., 1998, 51(1):3-32.
E.Kadic, T.J.Heindel, An Introduction to Bioreactor Hydrodynamics and Gas-Liquid Mass Transfer, 2014.
P.Malik, T.K.Mukherjee, Large-Scale Culture of Mammalian Cells for Various Industrial Purposes. Practical Approach to Mammalian Cell and Organ Culture, 2023.